Carbon4Energy
简介 We Provide Carbon-Based Chemical Solutions for Energy Conversion and Storage
分享到
Topotactic conversion of calcium carbide to highly crystalline few-layer graphene in water
2018
期刊
Journal of Materials Chemistry A
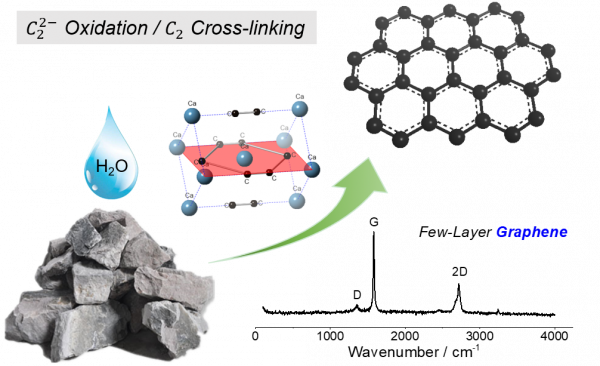
- 卷 6
- 期 46
- 页码 23638-23643
- Royal Society of Chemistry (RSC)
- ISSN: 2050-7488
- DOI: 10.1039/c8ta08632j
